‘Co-promotion’ increases between Korean Pharmaceutical Companies
As the research and development (R&D) capabilities of Korean pharmaceutical companies significantly improve and new drug development continues, the number of cases of marketing and sales collaboration, such as 'co-promotion' agreements executed for joint sales of drugs has been reported to be increasing.
It was reported on the 14th of this month, that a total of 14 cases of marketing and sales collaboration between Korean pharmaceutical companies occurred this year, through co-promotion agreements for joint sales of medicines.
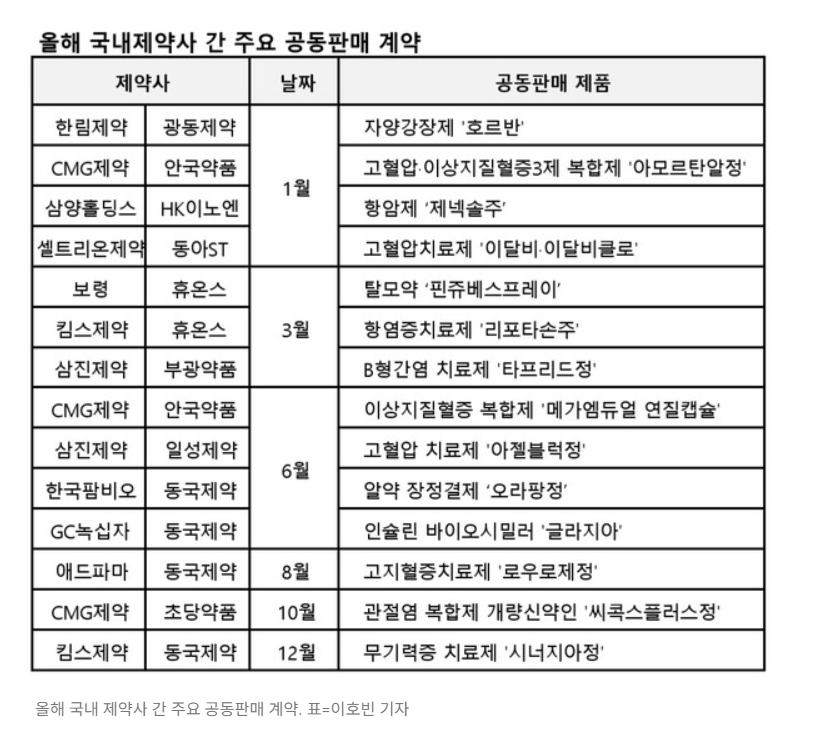
Co-promotion has the advantage of allowing developers with weak sales capabilities to leverage the capabilities of other pharmaceutical companies with strong sales capabilities in the field. One example is the joint sales of ‘Zemiglo’ by LG Chem and Daewoong Pharmaceutical, where at the time of approval, LG Chem signed a joint sales agreement with the global pharmaceutical company Sanofi-Aventis, but sales did not increase for three years. LG Chem collaborated with Daewoong Pharmaceutical which lead to increased sales resulting in the combined sales of the three Zemiglo series exceeding KRW 100 billion for the first time among Korean new drugs. It appears recently that the distribution rights for HK inno.N's 30th new drug in Korea, 'K-Cap', a treatment for gastroesophageal reflux disease, will be transferred from Chong Kun Dang to Boryung to increase profitability. Also, due to the strengthening CSO regulations, co-promotion or joint sales is becoming more popular among pharmaceutical and biopharma companies.
It would be recommended to carefully review and structure the co-promotion or collaboration agreements depending on the type of collaboration between the parties, and the type of drug or product, all based on strong negotiations of IP ownership occurring from the collaboration.





Comments
Post a Comment